How comorbidities affect SARS-CoV-2 viral entry
by Dr. Liji Thomas, MDAlmost from the start of the current COVID-19 pandemic, it has become clear that individuals suffering from other medical conditions like diabetes, cardiovascular disease, and lung disease are far more likely to contract the infection and to have a poorer outcome.
Now, a new study published on the preprint server bioRxiv* in May 2020 reports that such medical conditions influence the expression of genes that are linked to the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into the cell. The finding could have a significant bearing on potential therapeutic targets for COVID-19.
Pre-Existing Diseases Associated with A Higher COVID-19 Risk?
The link between the presence of these illnesses and the risk of acquiring this infection, or of having a worse outcome, is not clear. Does the pre-existing disease make the patient more vulnerable to the virus? Do they increase the virulence of the pathogen? Or is this apparent association the result of disease-specific factors, and as such a result of the general level of ill-health of the population at large?
Recent research shows that SARS-CoV-2 affects not just the lungs to cause pneumonia and acute respiratory distress syndrome (ARDS), but the heart, kidneys, and blood. This may cause the infection to progress into multi-organ dysfunction and compromise survival.
Protease Activation, Gene Expression, And Disease
SARS-CoV-2, like other coronaviruses, has spikes on its surface, composed of the spike (S) protein, which is a crucial component of viral entry into the host cell. This protein is required for the binding of the virus to the ACE2 receptor on the host cell membrane. Since ACE2 is part of the renin-angiotensin system (RAS) that modulates multiple vascular functions in the body, the genes that regulate these elements were brought into the current study.
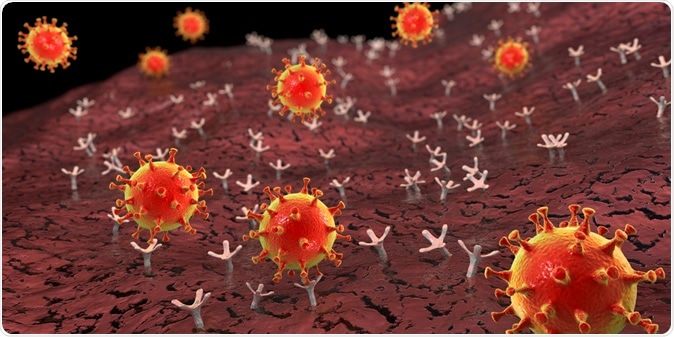
SARS-CoV-2 viruses binding to ACE-2 receptors on a human cell. Image Credit: Kateryna Kon / Shutterstock
Once the virus receptor-binding domain (RBD) binds to the ACE2 receptor, the S protein domain containing the RBD is cleaved off. This cleavage step facilitates viral fusion with the host cell membrane, which is what drives the virus into the cell. The protease involved in this protein cleavage may depend upon a change in the cellular endosomes or the activity of L-cathepsin, or via membrane-bound serine proteases found either at the surface of the host cell or inside vesicles.
Still, another mechanism of viral entry involves the splitting of the ACE2 molecule itself by such serine proteases, resulting in increased viral entry. The inhibition of serine proteases in vitro has underlined the major role played by these enzymes in viral entry.
The most well-known serine proteases in this context are TMPRSS2, and human airway trypsin-like protease (HAT or TMPRSS11D). However, the current study also includes other transmembrane proteases that can act the same way.
Finally, the researchers looked at how comorbidities could affect the expression of the major genes involved in the RAS and protease genes, so as to pave the way for viral entry into the cells of various tissues and organs.
The Study Design
The study included over 2,000 patients with hypertension, diabetes, obesity, chronic lung disease, cardiovascular disease, cancer, and chronic kidney disease. They used the data on gene expression from various gene databases such as the National Center for Biotechnology Information (NCBI), U.S. National Library of Medicine, Gene Expression Omnibus (GEO) DataSets and the European Molecular Biology Laboratory (EMBL), European Bioinformatics Institute (EBI). Differential analysis was carried out for each of these conditions.
The genes related to the RAS in health include ACE, ACE2, and AGTR1, which are transcribed from the DNA at varying levels. In addition, protease genes like ADAM17, TMPRSS1-5, TMPRSSD, TMPRSSE, and TMPRSS15 are also differentially expressed depending on the state of metabolism and health. This may help the virus to easily gain entry to the host cells across a spectrum of tissues.
The researchers observed that some organs like the heart, kidney, colon, and liver, as well as the lungs, expressed these genes abundantly, but not the adrenal, the bone marrow, and the ovary.
Again, the study examined the effects produced by many frequently co-occurring medical conditions like those named above on gene expression in some organ systems such as the lungs, heart, vascular and renal tissues.
Varying Effects of Coexisting Disease on Viral Entry
The expression graph shows that ACE2 expression increased most remarkably in cancer, along with almost every TTSP. This verifies their importance in cancer cell proliferation, motility, and invasion. Smokers also showed higher levels of ACE2 throughout their airways.
However, asthma patients did not show significant changes in the level of expression of these genes. Still, bronchial tissue was more likely to express ACE2 and TTSPs at higher levels than nasal epithelium.
In the kidneys, high ACE2 expression was most likely to occur in obese individuals. Smoking and cancer both increased ACE2 expression, as seen in the lung tissue, with a slight elevation of TTSPs. With hypertension, there were increases in the level of expression of ACE2, TMPRSS1 and TMPRSS4 within the kidney cortex and the tubulointerstitial tissue, though the glomeruli and medulla were spared.
The broadest range of expression levels was in chronic kidney disease, where TMPRSS4 was always higher in both glomerular and tubular tissue.
With heart disease, the highest level of ACE2 increase was in patients who had heart failure and diabetes, or who had aortic stenosis. The pattern was not so clear-cut in cardiomyopathies, but ACE2 was generally increased in the left ventricle and decreased in the right ventricle. Most of the TTSPs showed an insignificant increase.
In blood samples, coronary artery disease was associated with a dramatic increase in all the genes under study. With hypertension or chronic lung disease, there was a slight increase across the board. However, there was no modulation of the expression of any of the genes within the immune cells with obesity, unlike that seen in the kidneys.
Type I diabetes also was linked to a higher expression of these genes, but with type 2, both whole blood and white cells showed decreased gene expression. This was not seen with chronic kidney disease, where the blood levels of these genes were very variable.
The Way Ahead
The increases in the expression of ACE2 and TTSPs like TMPRSS2 genes in a range of tissues, in many commonly encountered conditions, should help to explain why they are linked to a higher rate of COVID-19 infections and death. On the other hand, the failure to observe a uniform pattern of alteration across tissues in obesity, diabetes, chronic lung disease, or cardiomyopathy, may be due to tissue-specific preferential viral entry.
It may thus be helpful to understand how differential gene expression in various organs targeted by the virus or impacted by co-existing chronic diseases influences the virulence of the SARS-CoV-2. The researchers sum up, “This represents an important step in designing effective therapeutic and preventative strategies to improve outcomes in vulnerable populations.”
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Breidenbach, J. D. et al. (2020). Impact of Comorbidities on the Expression of SARS-CoV-2 Viral Entry-Related Genes. bioRxiv preprint. doi: https://doi.org/10.1101/2020.05.26.117440. https://www.biorxiv.org/content/10.1101/2020.05.26.117440v1