An almost perfectly efficient light-activated catalyst for producing hydrogen from water
Efforts to make hydrogen from water directly using sunlight have been hampered by the inefficiency of the catalysts that promote the process. A model system demonstrates that almost perfectly efficient catalysts can be made.
by Simone PokrantSince the emergence of Greta Thunberg’s ‘Fridays For Future’ movement in August 2018, the need to prevent climate change and to find ‘green’ alternatives to fossil fuels have become topics of broad public interest. But although public awareness has advanced rapidly, progress in the search for cost-effective technological solutions has not. One promising sustainable energy carrier is hydrogen, if it can be produced using renewable energy sources — hydrogen is a green fuel, because its combustion produces only pure water. Writing in Nature, Takata et al. [1] report a breakthrough in catalyst design that might accelerate the development of large-scale processes for making hydrogen from water using sunlight.
The largest potential source of renewable energy is the Sun [2]: about 0.02% of the solar energy absorbed by Earth’s surface annually would be enough to cover current global energy consumption. Many approaches for converting solar energy into the chemical energy stored in hydrogen are therefore being investigated, using ‘water-splitting’ reactions in which water is broken down into hydrogen and oxygen. Some of these approaches are already being tested in pilot facilities — such as solar-power-to-gas units, in which electricity produced by solar cells is used to split water through electrolysis [3]. Hydrogen produced in this way could be used for the long-term storage of solar energy, or as fuel for vehicles. However, solar-to-gas conversion processes are not yet economically viable.
A study of the technical and economic feasibility of solar-energy production [4] has shown that systems based on light-activated catalysts (photocatalysts) are attractive alternative options for water splitting. In these systems, photocatalytic semiconductor particles are suspended in a bed filled with an aqueous electrolyte; when sunlight shines on the suspension, hydrogen and oxygen gases are produced. The technical simplicity of this approach should enable economically competitive hydrogen production, if the photocatalyst can convert solar energy to hydrogen with a minimum efficiency of about 10%.
However, the conversion efficiencies of photocatalytic semiconductors are typically much lower than 10%. This is because the photocatalytic process is highly complex and requires the semiconductor particles to have a combination of several properties. They must: absorb light; generate and separate electron–hole pairs (holes are positively charged quasiparticles produced when photons knock electrons out of an atomic lattice); enable holes and electrons to travel to the particle–water interface; and catalyse the production of hydrogen and oxygen from water (reactions that require electrons and holes, respectively). Side processes that can occur at each step can lower the overall conversion efficiency. Materials scientists are therefore trying to design photocatalysts that minimize such efficiency losses.
A key measure of the effectiveness of a photocatalyst is the fraction of absorbed photons that it can use to produce hydrogen, a quantity called the internal quantum efficiency (IQE). A perfect photocatalyst that converts all of the absorbed photons to hydrogen would have an IQE of 1 (or 100%). However, IQE cannot be determined from experiments.
A related quantity that can be experimentally determined for a reaction is the external quantum efficiency (EQE): the fraction of photons illuminating the reaction vessel that the photocatalyst can use to produce hydrogen. This value is always lower than the IQE, because an unknown portion of the illuminating photons will not be absorbed by the photocatalyst, but will instead be lost to other processes, such as scattering. If similar photocatalyst-particle suspensions are investigated using the same experimental set-up, ensuring that the same fraction of light is absorbed, then EQE can be used as an indirect measure of IQE. But EQEs determined using different set-ups cannot be used as a way of comparing IQEs of photocatalytic systems, because the relationship between EQE and IQE is different for each set-up — therefore making it difficult for different research groups to compare results.
Takata et al. focus on strontium titanate (SrTiO3) — one of the first materials found to split water photocatalytically, as reported [5] in 1977. Strontium titanate produces electron–hole pairs by absorbing ultraviolet light. Because the Sun’s intensity is highest in the visible-light range, it is unlikely that UV-driven catalysts will enable sustainable hydrogen production on a large scale. However, strontium titanate is an excellent model system for studying the influence of photocatalyst parameters on quantum efficiency (both EQE and IQE), because the mechanisms that cause efficiency losses in this material are well understood, and strategies for mitigating the losses have been proposed.
The authors used a combination of approaches to address specific loss mechanisms. One such mechanism is charge recombination, a process in which electrons and holes recombine before they can take part in water splitting. Takata and colleagues suppressed recombination in several ways. The first approach was to improve the crystallinity of the photocatalyst particles, thereby reducing the number of lattice defects. Another method was to reduce the number of chemical defects in the crystal lattice using aluminium doping — a process in which small quantities of aluminium atoms are incorporated into the lattice. These two approaches work because any defect (a lattice defect or a chemical defect) can act as a potential centre for recombination [6].
Takata and colleagues also took advantage of the fact that electrons and holes in their strontium titanate crystals collect at different crystal facets — a feature that further suppresses charge recombination. The authors selectively deposited appropriate co-catalysts on the facets to promote hydrogen production at the electron-collecting facets, and oxygen production at the hole-collecting facets (Fig. 1); this approach was previously proposed [7] and developed [8] by other research groups. Finally, the authors prevented an unwanted side reaction (the oxygen-reduction reaction) by encasing the rhodium co-catalyst for the hydrogen-producing reaction in a protective shell of a chromium compound.
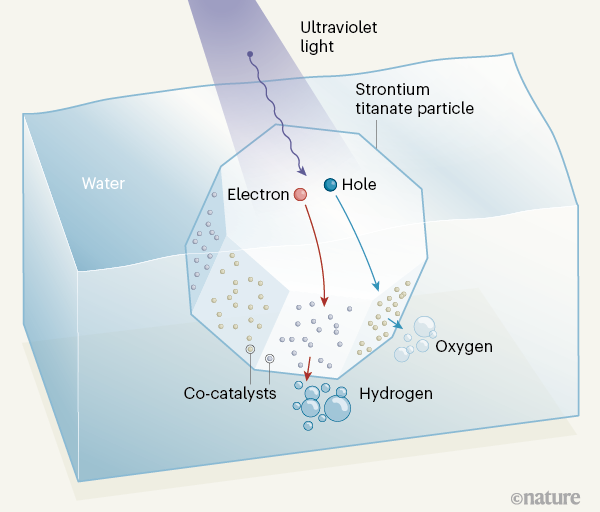
This combination of complex mitigation strategies proved highly successful: the authors reported EQEs of up to 96% when their photocatalysts were irradiated with light in the wavelength range of 350–360 nanometres. This is excellent news, because it means they have designed an almost perfect photocatalyst — the IQE must be between 96% and 100%.
This is a spectacular result for several reasons, even though strontium titanate is ‘just’ a model system for visible-light photocatalysts. First, it demonstrates that experiments can be designed in which EQEs come close to IQEs within an acceptable error margin of less than 4%. Improved experimental set-ups in which measured EQEs are very near to IQEs should facilitate the comparison of photocatalysts and therefore accelerate progress in this field.
Second, it proves that the combination of design strategies used by the authors can indeed eliminate efficiency losses associated with recombination. It is to be expected that the strategies used to improve the efficiency of strontium titanate will also apply to photocatalysts driven by visible light — and could therefore enable the conversion of solar energy to hydrogen with efficiencies of about 10%.
Finally, and most importantly, Takata and colleagues’ findings will inspire and encourage other researchers to continue their work on photocatalysts. One of the authors of the work, Kazunari Domen, published his first paper [9] on the use of strontium titanate as a photocatalyst in 1980. This shows the timescale needed for success in this area. Although we do not yet have a route for the sustainable and economically viable production of hydrogen, we stand a good chance of finding one in the next few decades. This paper vouches for it.
Nature 581, 386-388 (2020)
doi: 10.1038/d41586-020-01455-w
References
1. Takata, T. et al. Nature 581, 411–414 (2020).
2. Abbott, D. Proc. IEEE 98, 42–66 (2010).
3. Ochoa Robles, J., De-León Almaraz, S. & Azzaro-Pantel, C. in Hydrogen Supply Chains 8–11 (Academic, 2018).
4. Pinaud, B. A. et al. Energy Environ. Sci. 6, 1983–2002 (2013).
5. Wrighton, M. S., Wolczanski, P. T. & Ellis, A. B. J. Solid State Chem. 22, 17–29 (1977).
6. Ham, Y. et al. J. Mater. Chem. A 4, 3027–3033 (2016).
7. Giocondi, J. L. & Rohrer, G. S. J. Am. Ceram. Soc. 86, 1182–1189 (2003).
8. Mu, L. et al. Energy Environ. Sci. 9, 2463–2469 (2016).
9. Domen, K., Naito, S., Soma, M., Onishi, T. & Tamaru, K. J. Chem. Soc. Chem. Commun. 543–544 (1980).