Adamis Pharmaceuticals: Barely Holding On After Q1 Report
by BiologicsSummary
- Adamis recently reported their Q1 earnings with a miss on EPS and revenue. The COVID-19 pandemic has had a significant impact on the company's US Compounding sales.
- Adamis has decided to cut ties with their SYMJEPI commercial partner, Sandoz. The company was able to sign a U.S. commercial partnership with US WorldMeds, who is looking to push both SYMJEPI and ZIMHI.
- Despite the company's moves, I am barely holding onto my ADMP shares. I debate whether to buy, sell, or hold ADMP and reveal my plans for holding onto my position.
Once again, I find myself debating on whether to dump my Adamis Pharmaceuticals (ADMP) shares or to hold onto them in hopes the company can turn things around. The company reported another disappointing earnings report that revealed a miss on EPS and revenue. Admittedly, the company's poor Q1 performance was primarily due to COVID-19 and the nation’s lockdown measures. However, I was hoping to hear some optimism from the company’s c-suite during their earnings call but the call was lackluster despite a new US commercial agreement for SYMJEPI and ZIMHI. What is more, the company previously resubmitted ZIMHI’s NDA to the FDA… the call should have been filled with assurance and positive remarks. Instead, I felt a sense of hopelessness and the share price reacted accordingly. Once more, I filled my order to liberate myself from ADMP to only clear the forms and reassess the situation. What has changed? Isn’t the company in a better position now than a year ago? Is there any bullishness left in ADMP?
I intend to review the company’s recent updates and Q1 earnings to amass some evidence to determine whether to buy, sell, or hold my ADMP. In addition, I discuss my plans for ADMP position and the requirements I need to see to remain onboard.
Q1 Results
Adamis had a rough Q1 with their revenues coming in at $4.7M, which is a 4.9% drop from $4.9M in Q1 of 2019. The company attributes this decrease to COVID-19’s impact on sales from their subsidiary, U.S. Compounding. The company expects this state to persist in the near term as long as hospitals remain dedicated to COVID-19 patients and elective procedures are put on hold. In addition, the horse racing industry is currently shut down, so the demand for the company’s unique horse ulcer medication is much lower. The company did change gears to help the COVID-19 effort to start production of hand sanitizers, and the manufacturer of compounds that are in short supply, which could be a fruitful endeavor considering many hospitals are running low on the drugs needed to intubate COVID-19 patients and are lacking the API to produce in-house.
SG&A expenses were $6.1M and R&D expenses came in at $2M. Both of these numbers were down compared to the first quarter of 2019, due to the company reducing their R&D activities and payroll. Still, expenses are still pretty high for a company that is essentially treading water.
In terms of cash position, the company finished Q1 with approximately $10.5M in cash and equivalents as a result of a registered direct offering that provided net proceeds of roughly $6.2M. In April, the company did receive $3,191,700 under the Paycheck Protection Program “PPP” from the Coronavirus Aid, Relief, and Economic Security Act.
Adamis is looking to keep their cash burn to between $3M and $4M per quarter in 2020, which should be “sufficient operating capital to carry the company through 2020.”
APC-4000 Moving Forward
The company has conducted in-vitro proof-of-concept studies for dry powder inhaler “DPI,” APC-4000. Finally, Adamis should be able to press forward with clinical and commercial development. In the company’s Q1 conference call, the management mentioned they are “currently considering partnering opportunities for the future development and commercialization of APC-4000 as a product candidate and for the DPI inhaler platform as a whole,” which would probably be the best move for the product, company, and ADMP shareholders. I believe there is a lot of latent value in the company’s DPI platform and it’s obvious the company won’t be able to tap into that value in their current condition. Therefore, finding a partner that can maximize the technology should bring a healthy upfront payment, development milestone payments, and royalties.
It kind of reminds me of one of my neighbors, who had a partially restored ’68 ½ Mustang and had all the parts sitting in his garage. He knew how to fix it but he doesn’t own a lift or the specific tools needed to finish the job. So the car sat there unfinished despite its enormous value. My neighbor finally sold the classic car about two weeks ago to a shop in town who could finish the job. I don’t know the financial details of the transaction but it is nice to see the car finally getting the attention it deserves. I see the company’s DPI technology to be in the same situation. If the company can’t finish the job without excessive dilution and delay…find a partner who can.
Banking On ZIMHI
The company’s high-dose naloxone product, ZIMHI, was starting to look like SYMJEPI Saga 2.0. The company received a complete response letter (CRL) from the FDA back in November for primarily CMC issues. In December, Adamis submitted their responses to CRL and a request for a meeting. Back in February, Adamis met with the FDA to discuss the company’s responses and the specific information essential for an NDA resubmission. Adamis completed their enabling work and resubmitted the NDA this past week, so we should hear a decision about what type of resubmission it will be classified as in the next month or so.
A Class 1 response would be a 2-month review cycle, but a Class 2 response would involve a 6-month review cycle. Clearly, investors would like a Class 1 response, which would designate that the requested info was minor and would put the PDUFA in the middle of 2020. A Class 2 response would schedule the PDUFA in the second half of 2020 and would certainly have an undesirable impact on the share price. Unfortunately, the company can’t afford any bad news at this point for a number of reasons. Primarily, the share price continues to remain under $1 per share, which could force the company to perform a reverse split in order to regain NASDAQ compliance. In addition, the company will most likely have to raise cash at some point this year if Adamis fails to get ZIMHI approved and on the market. Essentially, I believe Adamis needs to “win-out” with ZIMHI in order to keep an ounce of bullishness left in the ticker.
US WorldMeds
At first, Sandoz appeared to be an amazing partner that was going to make SYMJEPI a legitimate contender against Mylan (MYL) and Pfizer’s (PFE) EpiPen. Unfortunately, Sandoz took forever to essentially come up with nothing and was not willing to commit to a field-based sales force. It was obvious that SYMJEPI was going to need a sales force to create some brand recognition and the institutional market appears to be committed to the cost-saving ampule route.
Thankfully, the company has publicized that Adamis and Sandoz had jointly agreed to return the marketing and distribution rights for SYMJEPI and SYMJEP Jr. to Adamis. Subsequently, Adamis stated that Adamis had signed a U.S. commercial partnership with US WorldMeds for both SYMJEPI and ZIMHI.
According to Adamis, “US WorldMeds has a long and successful history of developing and commercializing pharmaceutical products” and it was obvious that ZIMHI would be easy to promote alongside US WorldMeds' Lucemyra, which is a first-in-class treatment of opioid withdrawal symptoms after abrupt discontinuation. Adamis was also impressed with US WorldMeds’ history of “turning around products that had stalled under other organizations” and their plans to use a dedicated salesforce. What is more, US WorldMeds is already preparing to take over SYMJEPI, and Adamis’ CMO Dr. Moss is already involved in sales training with US WorldMeds in an attempt to get SYMJEPI in front of prescribers in advance of the back-to-school season.
My Thoughts On The Partnership
I feel like US WorldMeds is like a step-father for the company’s SYMJECT products and for ADMP investors. Sandoz wasn’t providing the support needed to make SYMJEPI a household name and they weren’t willing to change their approach. Consequently, Dr. Carlo had to terminate their agreement and find another partner who was willing to take on SYMJECT products as their own and listen to Adamis. But I am left wondering… who are they? I was hoping for a name brand partner but I am guessing most of those suitors are moving on to other prospects or endeavors. Now, investors have been presented a relatively unknown company that will be responsible for essentially turning the company around. Admittedly, I wasn’t exactly overjoyed about the announcement, but once I found out that they push Lucemyra, I understood why Dr. Carlo went with US WorldMeds. Despite my previous stipulations, I am going to give US WorldMeds a chance to push SYMJEPI and see if they are able to beat Sandoz’s SYMJEPI numbers in the first couple of quarters. Indeed, outperforming Sandoz’s effort won’t be too difficult, but any improvement should be seen as a bullish indicator.
On the other hand, if US WorldMeds cannot outperform Sandoz in the first two quarters of handling commercialization, I will concede that SYMJEPI is not a legitimate competitor to epinephrine auto-injectors.
Buy, Sell, Or Hold
I am finding it harder and harder to make a decision on what to do with my ADMP shares. It feels as if every time I contemplate pressing the sell button, I re-evaluate the company’s current value and potential for growth. At the moment, Adamis has an attractive price-to-sales valuation and the company should see an increase in the revenue in the coming years as ZIMHI hits the market and US Compounding continues to report solid growth (Figure 1).
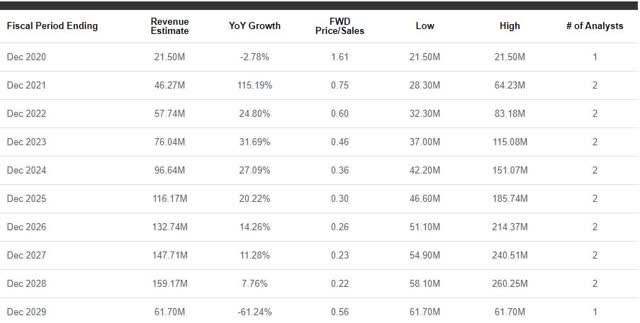
Figure 1: ADMP Annual Revenue Estimates (Source: Seeking Alpha)
Another reason why I have decided to hold my ADMP shares is the technicals. The stock has been hanging out in the $0.50 area for an extended period of time and has broken out of most long-term downtrends (Figure 2).
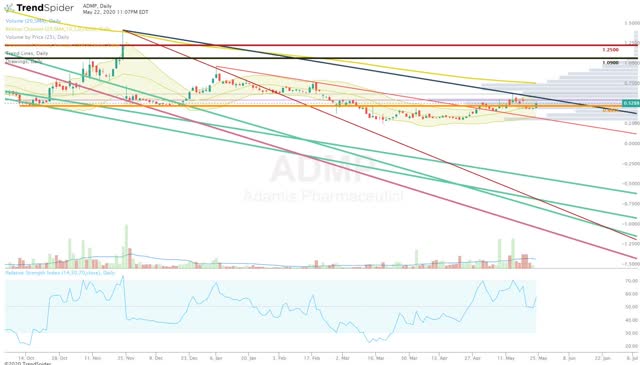
Figure 2: ADMP Daily (Source: Trendspider)
Essentially, I haven’t sold because I still see the potential upside and the share price has slowed its deterioration.
However, I haven’t found a reason to click the buy button since ZIMHI’s CRL. SYMJEPI has had an abysmal launch and the company continues to run secondary offerings almost twice a year. Indeed, some of the circumstances are out of the company’s hands. However, I am not willing to commit to reloading my position until ZIMHI is approved and US WorldMeds has reported an improvement in SYMJEPI sales.
Verdict
Adamis continues to be one of my masochist investments that have become a do-or-die in 2020. I need the company to win out in 2020, with a Class 1 response, FDA approval of ZIMHI, improved commercial numbers for SYMJEPI, and US Compounding is able to get back on track towards profitability. In addition, I can’t deal with another offering in the near future, so, I have to see the company make it into 2021 before they sign the papers for someone to run another secondary. Finally, I won't stick around if the company plans on performing a reverse split. Despite my disgruntled views of the management and the company’s history, I still find ADMP worthy of speculative buy for a position trader who is looking to get in ahead of ZIMHI’s PDUFA, or a long-term investor looking to buy some cheap lotto tickets.
Disclosure: I am/we are long ADMP. I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.