Toxin family binds to sugar receptors on human cells to cause damage
by Science X staff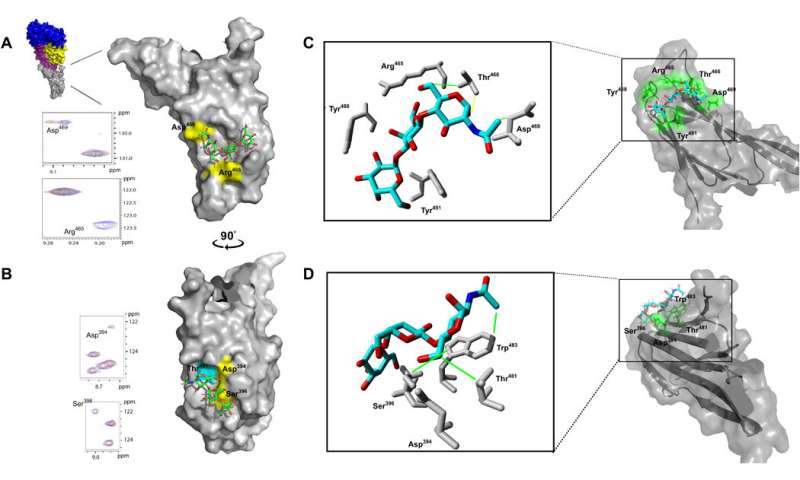
New Griffith University research has found that sugars decorating human cells allow toxins, produced by disease-causing bacteria, to bind to human cells and cause damage or death.
The family of toxins known as cholesterol-dependent cytolysins (CDCs), are produced by bacteria such as Streptococcus pneumoniae, group A Streptococcus and Listeria monocytogenes, which cause pneumonia, invasive group A Strep disease (including what is known as flesh-eating disease) and listeriosis.
These toxins damage human cells by forming pores in the membrane which cause the cells to break open.
In a paper published in Science Advances today, the Institute for Glycomics researchers leading an Australian and international team found found that cell specific sugars are the targets for eight major CDCs and not only cholesterol, as previously thought.
Lead researcher Dr. Lucy Shewell said they could stop the toxins causing damage to human blood cells by adding in the binding sugars.
"By adding sugars in solution with the toxins, the toxins are effectively mopped up before they do any damage to the red blood cells.
"The CDCs still need cholesterol in the membrane to allow them to form the final pore, but they need to bind to the sugars to recognise the cells they kill,"' Dr. Shewell said.
"Our research has shown that it is actually specific sugars on the cell surface which are the binding targets for these toxins. We have also identified the part of the toxin responsible for sugar binding.
"Understanding to which sugars these toxins bind gives us greater insight into which cells they damage and can allow us to develop therapeutics to block their harmful action."
Corresponding author of the study, Professor Michael Jennings said, "This research provides new information that will guide development of small molecule drugs and new vaccines that targets these toxins."
Professor Mark von Itzstein AO, Director of the Institute for Glycomics, said this was another excellent example of how the Institute's unique research approach centred around 'glycomics' can pave the way to new translational outcomes in their ongoing fight against some of the most debilitating diseases.
More information: Lucy K. Shewell et al. All major cholesterol-dependent cytolysins use glycans as cellular receptors, Science Advances (2020). DOI: 10.1126/sciadv.aaz4926
Journal information: Science Advances
Provided by Griffith University