Transparent human organs allow 3-D maps at the cellular level
by Science X staff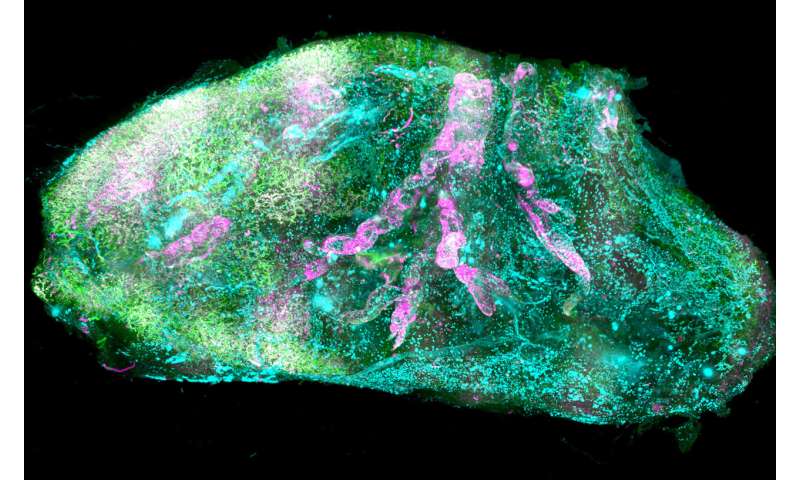
For the first time, researchers have managed to make intact human organs transparent. Using microscopic imaging, they revealed complex underlying structures of the transparent organs at the cellular level. The resulting organ maps can serve as templates for 3-D bioprinting technologies. In the future, this could lead to the creation of on-demand artificial organs for many patients in need. The findings have been published in Cell.
In biomedical research, seeing is believing. Deciphering the structural complexity of human organs has always been a major challenge due to the lack of technologies to image them at the cellular level. Recent developments in tissue clearing haver allowed researchers to obtain the first cellular views of intact transparent mouse organs in 3-D. These methods, however, were not applicable to human organs.
Human organs are particularly stiff due to the accumulation of insoluble molecules, including collagen in tissues that have grown for years or even decades. Thus, traditional detergents that are used for making mouse organs transparent do not work on human organs, particularly adult ones. "We had to change our approach completely and start from scratch to find new chemicals that can make human organs transparent," says Shan Zhao, Ph.D. student at Helmholtz Zentrum München and first author of the study. After exhausting trials, the team discovered that a detergent called CHAPS could make small holes throughout the entirety of stiff human organs. CHAPS allows additional solutions to travel deep into centimeters-thick human organs and convert them into a transparent structure.
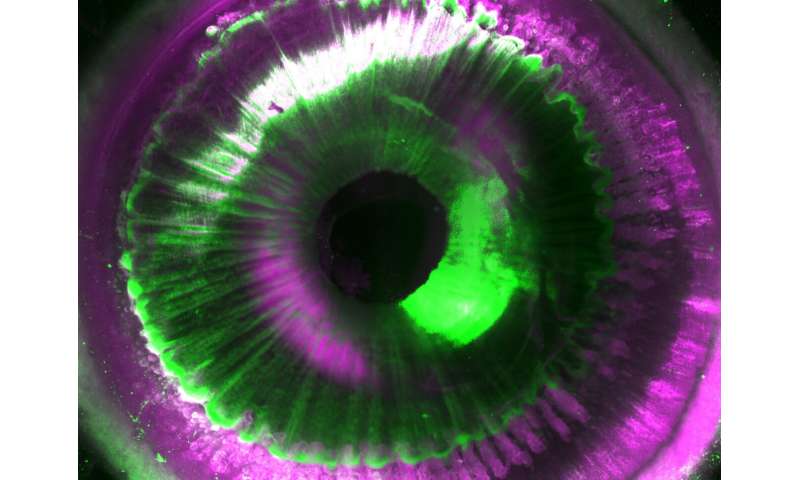
After making postmortem human organs transparent, the team had to tackle additional challenges for both organ imaging and the analysis of the large amount of resulting data. First, they developed a new laser-scanning microscope called Ultramicroscope Blaze, which has a large sample holding capacity. This microscope enabled imaging of human organs as large as the kidney. Next, together with Prof. Bjoern Menze from TUM, the team developed deep learning algorithms to analyze hundreds of millions of cells in 3-D.
The researchers named this new technology SHANEL, which stands for Small-micelle-mediated Human orgAN Efficient clearing and Labeling. "SHANEL can develop into a key technology for mapping intact human organs in the near future. This would dramatically accelerate our understanding of organs such as the brain, their development and function in health and disease," explains Dr. Ali Ertürk, Director of the Institute for Tissue Engineering and Regenerative Medicine at Helmholtz Zentrum München and principal investigator at the Institute for Stroke and Dementia Research at the hospital of LMU.
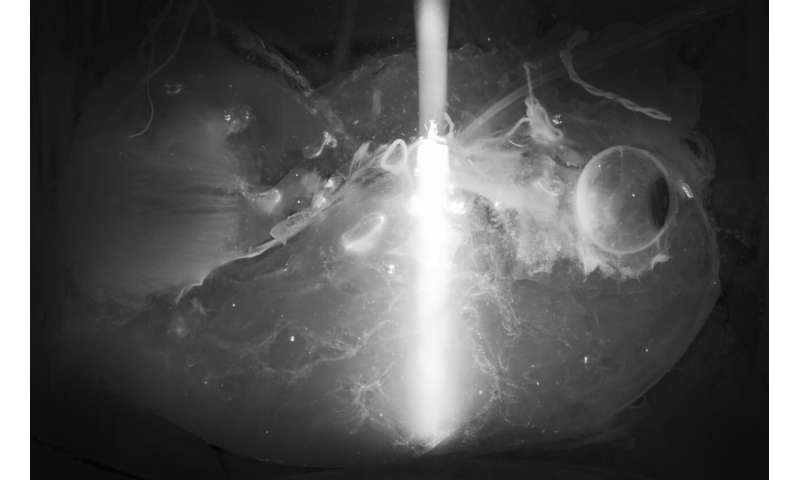
Final goal: 3-D-bioprinting of artificial organs
Cellular maps of human organs could be used to engineer large-scale human tissues and organs with emerging 3-D-bioprinting technologies. Toward this goal, Ertürk and his team are currently working on mapping major human organs, starting with the pancreas, heart and kidney.
"There is a huge shortage of organ donors for hundreds of thousands of people," says Ertürk. "The waiting time for patients and the transplantation costs are a real burden. Detailed knowledge about the cellular structure of human organs brings us an important step closer to creating functional organs artificially on demand."
More information: Shan Zhao et al, Cellular and Molecular Probing of Intact Human Organs, Cell (2020). DOI: 10.1016/j.cell.2020.01.030
Journal information: Cell
Provided by Helmholtz Association of German Research Centres