Measurement of mechanical stability of force transmission supramolecular linkages
by Science X staff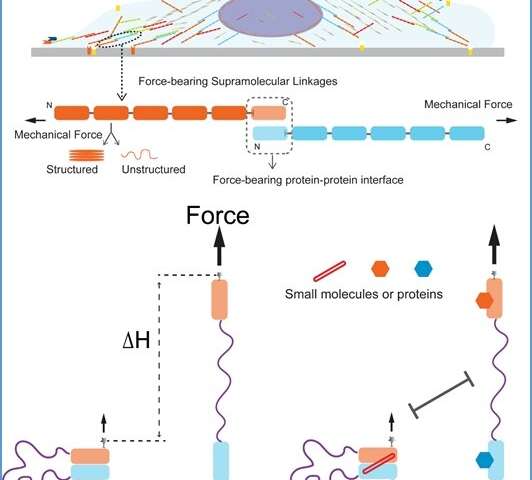
NUS biophysicists have developed a manipulation assay that can quantify the mechanical stability and biochemical regulations of inter-molecular interactions at the single-molecule level.
Mechanotransduction is a critical physiological process by which cells sense mechanical stimuli and translate them into biochemical and biological reactions. This process is responsible for a number of senses in the body, including touch, balance and hearing. Cells use an assembly of various supramolecular force-transmission linkages for mechanotransduction. A linkage typically comprises a few non-covalently linked proteins which are subjected to intracellular forces. By probing the mechanical and biochemical regulations of these force-transmission linkages, we can better understand the molecular mechanisms involved that allow cells to react to external changes.
The research team comprising Prof Jie YAN from the Department of Physics and Mechanobiology Institute, NUS, and his research fellows, Dr. Shimin LE and Dr. Miao YU, has developed a manipulation assay that enables direct measurement of the mechanical stability and biochemical regulations between protein molecules under different environmental conditions. Using this assay, they have systematically investigated several inter-molecular interfaces that play crucial roles in cell mechanotransduction. Their research results show that there is surprisingly high mechanical stability in these interfaces. This stability allows for the proper functioning of cellular functions involving force transmission at the molecular level.
The manipulation assay is like a long flexible string to tether the two molecules being measured. The two molecules are adhered to each other (paired state) in the initial state. When the inter-molecular interaction is ruptured under mechanical force, the molecules become separated (unpaired state). The separated molecules are kept in vicinity to each other by the flexible string, allowing them to re-pair after force is decreased. The separation of the molecules causes a large stepwise change of the extension of the molecule along the force direction, which can be measured (see Figure). Using this method, the mechanical stability of the interface between the two molecules can be quantified.
Prof Yan said, "Previous efforts to understand the molecular mechanisms underlying mechanosensing have mainly focused on understanding individual proteins that constitute the linkages and the proteins that interact with these linkages. However, the roles of the force-transmission linkages which connect these proteins together have largely remained unexplored. By focusing on these force transmission linkages, we can obtain a more systematic view of the mechanosensing mechanisms within a cell. This may also lead to the development of new approaches to modulate mechanotransduction which targets these linkages."
"The single-molecule assay developed in these studies can be extended to quantify the mechanical stability of any force-bearing inter-molecular interfaces. It can also potentially be used to search for pharmaceutical compounds that may alter the mechanical stability of selected intermolecular interfaces," added Dr. Le.
More information: Shimin Le et al. Phosphorylation Reduces the Mechanical Stability of the α-Catenin/ β-Catenin Complex, Angewandte Chemie International Edition (2019). DOI: 10.1002/anie.201911383
Miao Yu et al. Force-Dependent Regulation of Talin–KANK1 Complex at Focal Adhesions, Nano Letters (2019). DOI: 10.1021/acs.nanolett.9b01732
Shimin Le et al. Direct single-molecule quantification reveals unexpectedly high mechanical stability of vinculin—talin/α-catenin linkages, Science Advances (2019). DOI: 10.1126/sciadv.aav2720
Journal information: Angewandte Chemie International Edition , Nano Letters , Science Advances
Provided by National University of Singapore