New cobalt complex could reduce the cost of hydroformylation
by Bob Yirka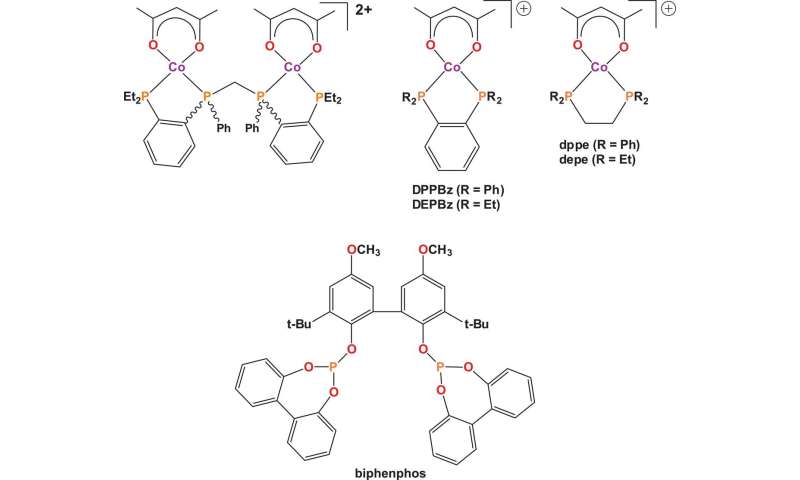
A team of researchers from Louisiana State University and ExxonMobil Chemical has found a way to replace the rhodium catalysts used in the hydroformylation process. In their paper published in the journal Science, the group describes their process and how it compares economically with current methods.
Hydroformylation is an industrial process used to produce the kinds of aldehydes that are heavily used in the petrochemical industry. Currently, rhodium catalysts are used in the process, but oil companies such as ExxonMobil would like to find a replacement due to their high cost—it currently sells for over $10,000 an ounce. In this new effort, the researchers claim to have found a viable alternative—a cobalt complex.
Cobalt was originally used to transform olefins into aldehydes for use in applications such as making petrochemicals, but they were replaced over time by rhodium catalysts. This was because they were much more active, which translated to faster reaction times. Thus, any replacement, including cobalt would need to have similar reaction times.
Cobalt is a chemical element found in the Earth's crust in a chemically combined form. Its use in industrial applications is primarily in lithium-ion batteries and magnets. And it is cheaper to buy than rhodium, but such prices are not a certainty. Approximately 66 percent of available reserves are in the Democratic Republic of the Congo, a historically unstable country. The researchers believe it is worth gambling on cobalt, however, at least for now, because its price is currently just 0.01 percent that of rhodium.
The new approach involves dissociating the carbonyl ligand from the catalyst, making space for the alkene and then applying a positively charged cobalt species—from there, the reaction is allowed to proceed until it finishes. The researchers used the positively charged cobalt species because it was believed to increase reaction times—the cationic charge on the cobalt would force the metal d-orbitals to contract, making the carbonyls more labile. Timing of test reactions showed that unlike prior efforts to use cobalt as a catalyst, it was relatively fast—within a factor of 20 when compared to reactions using rhodium catalysts.
More information: Drew M. Hood et al. Highly active cationic cobalt(II) hydroformylation catalysts, Science (2020). DOI: 10.1126/science.aaw7742
Journal information: Science
© 2020 Science X Network