Blue light activates antibodies on demand
by Samuel Vennin
Korean researchers have used light to control the binding of two separate and inactive antibody fragments and generate a specific, timely immune response to antigens. The platform that they created could help develop new therapies for cancer or autoimmune diseases (Nat. Methods. 10.1038/s41592-019-0592-7).
When a pathogen such as a bacteria or virus enters the human body, the immune system reacts by creating antibodies to identify the intruder and kickstart the chemical process that leads to its neutralization. With their Y shape, antibodies bind to a specific antigen and alert nearby phagocytes to the presence of an invader that needs to be eliminated.
Making antibodies more selective and efficient at detecting antigens has been a key research goal for developing new cancer or autoimmune therapies over the last three decades. Such antibodies, which have been engineered to generate a better and immediate immune response against their target antigens, are known as therapeutic antibodies. For example, the CAR T-cell cancer therapy that garnered a lot of attention following the 2018 Nobel Prize in Medicine associates a tumour-detecting antibody with cancer-killing T cells to create a “living drug” that can fight off tumours.
But for all their advantages, therapeutic antibodies are limited by our ability to control them. Their expression and regulation can be chemically induced, but the ability to precisely fine-tune their activity when and where needed has remained so far elusive, preventing the control of an antibody’s function within a living cell.
A one-in-all platform
A team led by Won Do Heo and Byung Ouk Park, from the Institute for Basic Science and Korean Advanced Institute of Science and Technology in South Korea, has now managed to achieve this control, by using a split–rejoin technique. Briefly, the researchers injected antibodies as two inactive split fragments (two separate branches of the Y shape) and used blue light to stimulate a reaction that led to linking of the branches and consequently, activation of their defensive function. They called the antibodies generated in this way “optogenetically activated intracellular antibody”, or “optobodies”.
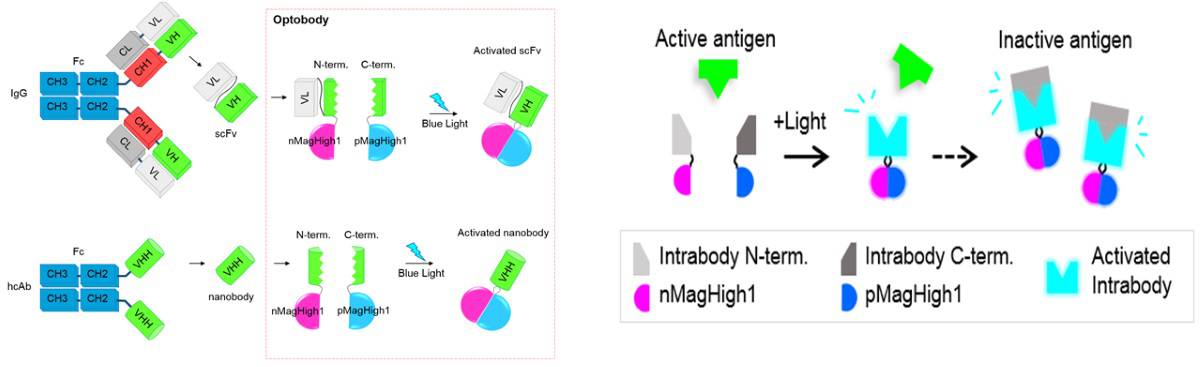
The researchers first optimized their platform for insertion of binding domains. They tested it on two antibody fragments selected for their high target-specificity and stability: a single-chain variable fragment (scFv) and a single-domain antibody (a so-called nanobody). They first identified in a green fluorescent protein (GFP) nanobody the sites that required optical stimulation to spur the reassembling of the antibodies, and then compared the mitochondrial activities of the optobodies with similar, unmanipulated, antibodies.
While each separated fragment of the antibody did not display much mitochondrial activity, the optobody generated by linking the two branches displayed similar expression patterns to the original antibody. This finding indicates that there is no functional difference between optobodies and the natural antibody they are replicating.
Towards future breakthroughs?
Finally, the researchers tested whether the platform could generate optobodies targeting specific cells to disrupt pathway signalling. They administered novel nanobody fragments to cells derived from mouse and human embryos and monitored the ability of the optobodies to inhibit specific target endogenous proteins in these living cells. The team paid close attention to the cell movement and receptor signalling, two characteristics of pathogen expression. All of the optobodies studied bound to their target proteins and induced a reduction in cell movement, along with a significant reduction of signalling transduction.
“Our optobody system is a great tool to study the role of endogenous proteins in living cells and animals, and also shows great clinical promise for therapeutic strategies in the future,” says Heo. This could prove all the more interesting because the trigger source is not limited to blue light – other wavelengths such as near-infrared light could provide similar results with a different pool of antibodies. By offering more precise control of target protein activity, both spatially and temporally, the technique could eventually lead to the design of inducible “living” drugs for conditions where current therapies remain ineffective.